St. Joseph’s Health Centre

30 The Queensway,
Toronto, ON
416-530-6000
Quick Links
CIBC Just for Kids Clinic
Emergency
Family Health Team
Medical & Diagnostic Imaging
Mental Health & Addictions
Obstetrics & Women’s Health
About us
St. Joseph’s Health Centre is a community academic hospital serving Toronto’s west end and beyond. The hospital is a vital resource for people at all life stages, offering obstetric care, specialized paediatric services, family medicine, seniors programs and more.
St. Joseph’s is one of three main sites of Unity Health Toronto, a Catholic health care organisation accredited with exemplary standing by Accreditation Canada. Unity Health’s services span the City of Toronto and range from primary care to highly specialized care for complex medical cases, post-acute care, rehabilitation, palliative and long-term care. We advance excellence in health care through education, research and innovation.
![]()
Construction Alert: Please be advised that the City of Toronto and TTC are currently doing construction work around St. Joseph’s. Please plan ahead for your appointment. For more information about this project please visit: www.toronto.ca/kqqr
Who we are
The best care experiences, created together
Emergency
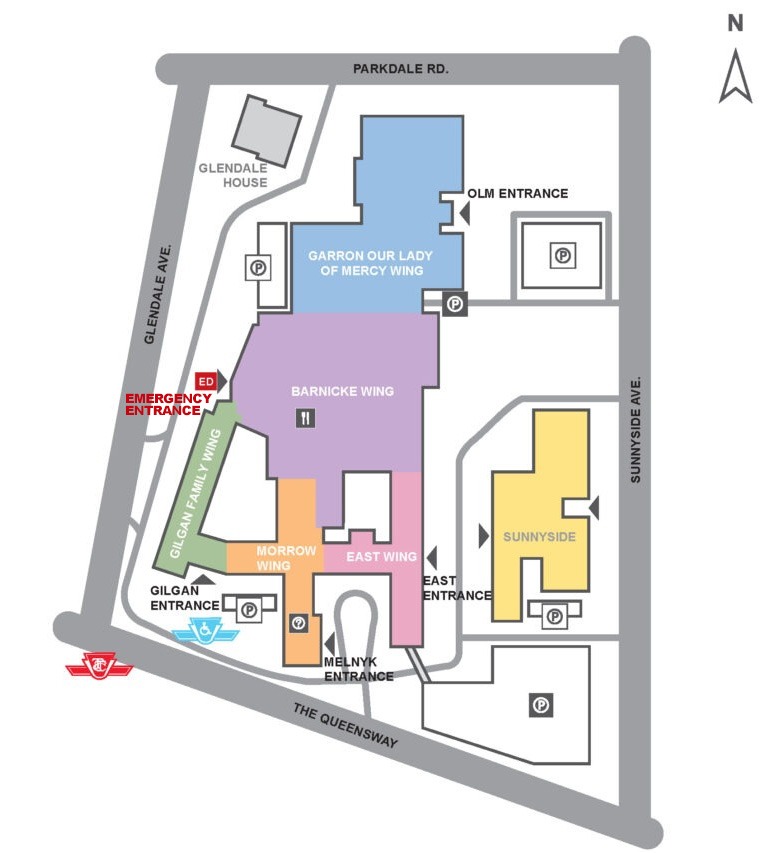
The Emergency Department is located on the ground floor in the Barnicke Wing, just inside the Emergency entrance.
Parking
- The main parking garage is located on Sunnyside Ave.
- Limited underground parking is available in the Our Lady of Mercy (OLM) Wing located off Sunnyside Avenue (just north of the main garage).
- There is some surface parking near the Emergency Department. To access this parking area, please use the main Emergency driveway off Glendale Ave.
On-Site Directions
Keeping Our Site Clean
St. Joseph’s is located in the heart of Roncesvalles, just steps from some of the city’s most vibrant residential and commercial streets. Whether you’re visiting the site to work, learn, receive care or visit a loved one, it’s important that we treat the site and community with kindness and respect. One way to do this is by keeping the neighbourhood clean. Please be mindful of your garbage and dispose of the following items properly:
- Cigarette butts go in cigarette butt disposals on City of Toronto garbage cans
- Masks and gloves go in garbage bins
- Pop cans go in recycling bins
- Organic waste should be placed in compost bins
Thank you for helping us keep Unity Health and our surrounding neighbourhoods clean. To learn more about how to dispose of various materials, please use the Waste Wizard on the City of Toronto website.
News and Updates

Last updated April 22, 2024